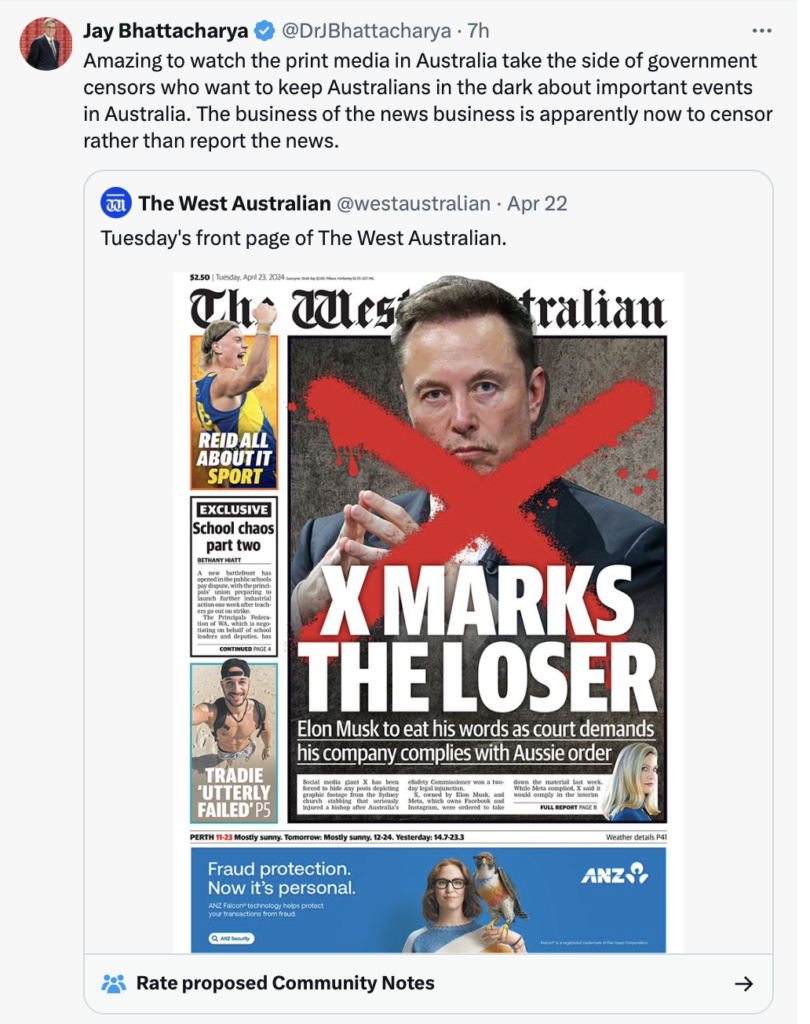
Now that signals are pouring in on the VAERS system (Vaccine Adverse Event Reporting System) that the COVID vaccinations are temporally associated with deaths and injuries, the Gates Foundation shows up right on schedule to attack the integrity of VAERS, warning of "Misinformation and the Vaccine Adverse Event Reporting System."
Dr. Peter McCullough explains his concerns:
Every week in clinic I make entries into the US CDC Vaccine Adverse Event Reporting System (VAERS) as I catch up on years of injuries, disabilities, and deaths that have occurred after vaccination. Because federal fines and penalties are severe for false reporting, I only enter cases in which I have the vaccine card, the full clinical vignette, and my clinical impression that the vaccine either directly caused the problem or significantly contributed in the causal pathway to the new disease or injury suffered by the patient. I did a PUBMED search today and there are > 500 papers that have relied on VAERS for epidemiologic studies of vaccine side effects including death in 126 manuscripts.
As the data mount, it should come at no surprise that the Gates Foundation, a major player in the Bio-Pharmaceutical Complex has come out with an attack on the integrity of VAERS. It came through a JAMA editorial from Kathleen Hall Jamieson, PhD, that implied VAERS is “misinformation” in the title uses the adjective “unverified.” Nothing could be further from the truth. Jamieson, whose Annenberg Center is funded in part by the Bill and Melinda Gates Foundation, goes on to propose a name change to VAERS to further diminish its importance. . . I expect at some moment, the data on vaccine safety will be so overwhelming in VAERS that the CDC will simply shut down access the system for queries and research. As sponsors of the program, the agency will refuse to tell America or the world anything on the safety of the novel, genetic biological products.
For the record, U.S. public health officials have used and trusted the VAERS system for decades. From the current VAERS About Page:
About VAERS
Background and Public Health Importance
Medical professionals working with vaccines
Established in 1990, the Vaccine Adverse Event Reporting System (VAERS) is a national early warning system to detect possible safety problems in U.S.-licensed vaccines. VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). VAERS accepts and analyzes reports of adverse events (possible side effects) after a person has received a vaccination. Anyone can report an adverse event to VAERS. Healthcare professionals are required to report certain adverse events and vaccine manufacturers are required to report all adverse events that come to their attention.
VAERS is a passive reporting system, meaning it relies on individuals to send in reports of their experiences to CDC and FDA. VAERS is not designed to determine if a vaccine caused a health problem, but is especially useful for detecting unusual or unexpected patterns of adverse event reporting that might indicate a possible safety problem with a vaccine. This way, VAERS can provide CDC and FDA with valuable information that additional work and evaluation is necessary to further assess a possible safety concern.
Let's see whether this self-description of VAERS, or even fundamental aspects of the VAERS system, changes in response to new suspicious pressures . . .